Bridge Phenomenon of Conductive Pollutants in Circuit Boards and Its Improvement
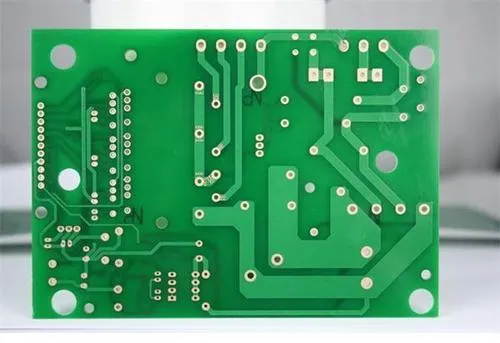
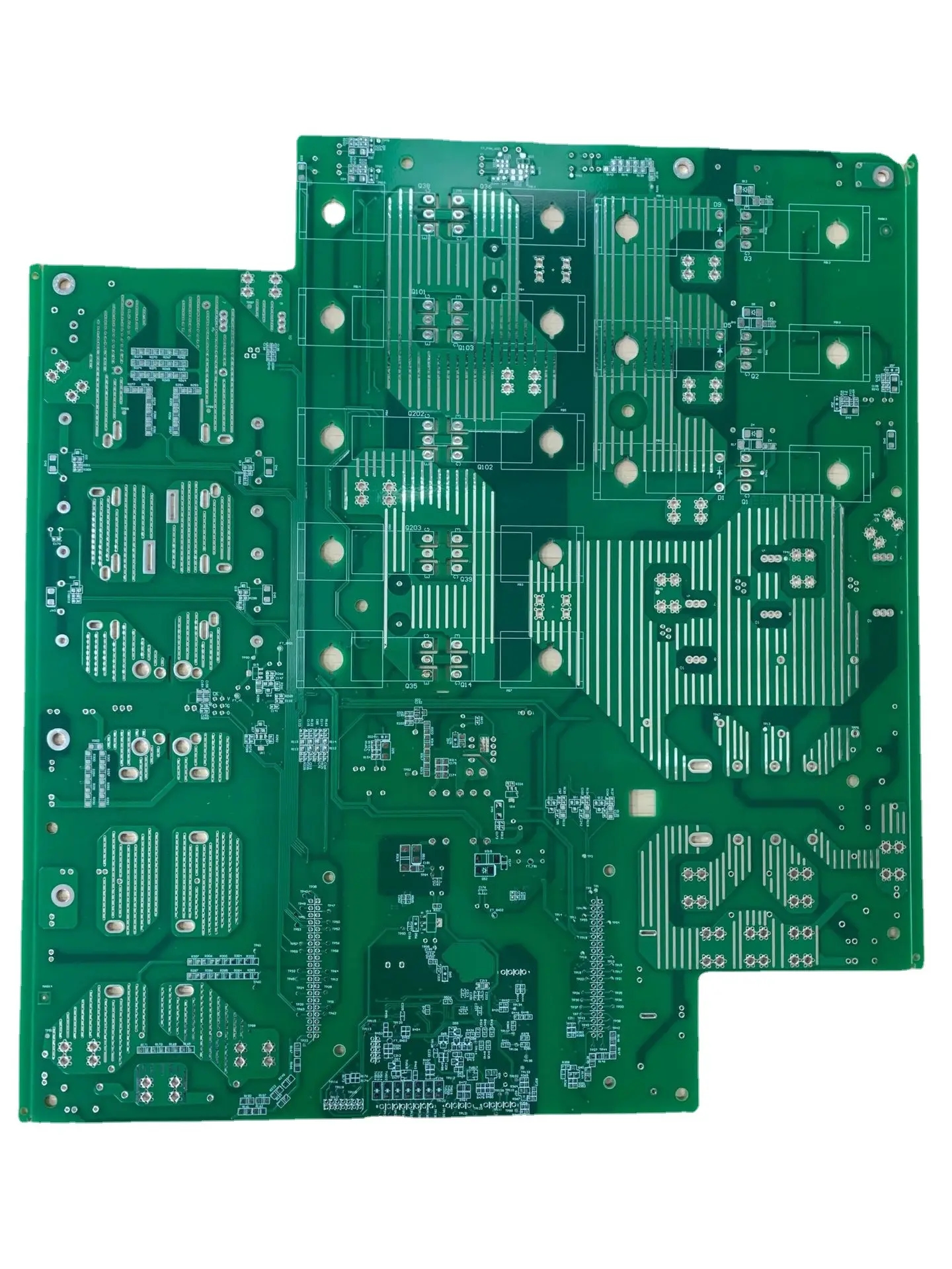
The bridging circuit formed by conductive salts may occur in the condition of circuit board electroplating, circuit board etching or the residual flux on the circuit board. These ions are good conductors in a wet environment. They will cause ion migration between the two conductors and create a short circuit on the surface of the insulator. Corrosive by-products, such as chlorine and sulfur salts, will form in the production environment. They are a chEMIcal type and may cause short circuit. An example of this type of failure is shown in the following figure. The occurrence of tree growth is due to the electrICal transfer of metals from one conductor to another, so it is also described as the diffusion and migration of electrical metals, and the tree growth fault example.
As long as the following conditions are met, the dendrite like crystals will form on the surface (including the inner surface of the cavity):
? Persistent liquid water film with a thickness of more than a few molecules
? Exposed metals, especially tin, lead, silver or copper, which may be oxidized at the anode
? Electrical bias of low DC
As long as there are hydrolytic ionic pollutants (such as halogen and acid released from flux residues or polymers), this phenomenon will be significantly accelerated. Explosion plate peeling or cavitation will also increase the accumulation of moisture or pollutants, which may increase the growth of the tree structure. The growth of conductive anode filament is a special case of tree structure growth, which will be discussed later. The time of fault occurrence is inversely proportional to the square distance and voltage. The fault mechanism in this regard has been reviewed in the accelerated test.
The growth of the tree is often from cathode to anode, and the formation of metal ions is generated by anodic dissolution, and then transported along the conductive path and reduced to the cathode. This precipitate looks like a branch because it has spreading branches. When this growth object touches another conductor, there will be an unexpected current rise there. SometiMES, it will damage the dendritic structure, but it may also cause a circuit to temporarily lose its function or damage the components. The proposed failure mode is that when moisture is absorbed, an electrochemical battery will be generated. Subsequent copper electrode reaction

example:
Anode: Cu - Cu++ne
H2O — 1/2 O2 + 2H+ + 2e_
Cathode: H2O+e-1/2H2+OH-
The main reason for electric leakage here is that due to the electrolysis of water, the copper metal diffuses and migrates to the cathode at the same time when it is dissolved in the anode. When it reaches the cathode, it is no longer soluble. The formation of branch structure follows the gradient of pH value. The voltage difference between the cathode and anode will also affect the growth rate of the tree structure. When the cathode and anode are the same metal (such as copper), although the intervention of moisture and air also has some effects, the initial voltage difference is mainly determined by the applied bias voltage. Corrosion may be accelerated due to cracks, or it may occur between the cathode and anode due to the difference of oxygen concentration. When metals are different, redox corrosion may occur without bias.
If there is an applied bias, and the yin and yang poles are in a watery environment, tree growth will almost happen immediately. A SIMple experiment can prove this view. As long as a 6-V bias voltage is applied between two conductors, it is enough to induce rapid growth (which can be observed with a low magnification microscope). Even in distilLED or deionized water, the conductor will still bridge, and it will be faster in tap water.
Redox corrosion can occur between dissimilar metals because they have different electron affinities (that is, they have high and low electronegativity tendencies). When metals are close to each other, the cathode behavior of the more noble metal end will be the anode, and moisture is an essential element for coupling the electrical properties of the two metals. Generally, it is not necessary to apply bias voltage, but if the polarity is correct, this reaction may be accelerated. When the anode is very SMAll compared with the cathode, its corrosion may be very fast. On the contrary, if the anode is much larger than the cathode, the corrosion may not be serious, especially if the potential difference is small.
The circuit board manufacturer and circuit board designer will explain the bridging phenomenon and improvement of conductive pollutants in circuit boards.
然后
聯系
電話熱線
13410863085Q Q
微信

- 郵箱